Abstract
Introduction: Ibrutinib is an irreversible inhibitor of Bruton's tyrosine kinase which has become an important part of the therapeutic armamentarium for B-cell malignancies. Ibrutinib has been associated with an increased incidence of atrial and ventricular arrhythmias in studies. We conducted an updated systematic review and meta-analysis of all phase III randomized controlled trials (RCT) to determine the relative risk of atrial fibrillation (AF) associated with ibrutinib use, relative risk of high grade AF (grade 3-5), and to evaluate if the estimation of risk has changed with emergence of new data since prior reports on this adverse event.
Methods: We performed a systematic search of PubMed, Embase, Web of Science, Scopus, Clinical Trials.gov, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews with appropriate keywords through 07/10/18, to find all RCTs comparing ibrutinib with other agents or placebo in patients with B-cell malignancies and also reporting AF as a treatment-emergent adverse event. The search strategy, study selection, data extraction, and analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines. We pooled the point estimates using random effects model of the generic inverse-variance method described by Der Simonian and Laird. Statistical analyses were performed using the Stata/SE 15.1 (StataCorp LP, College Station, TX, USA).
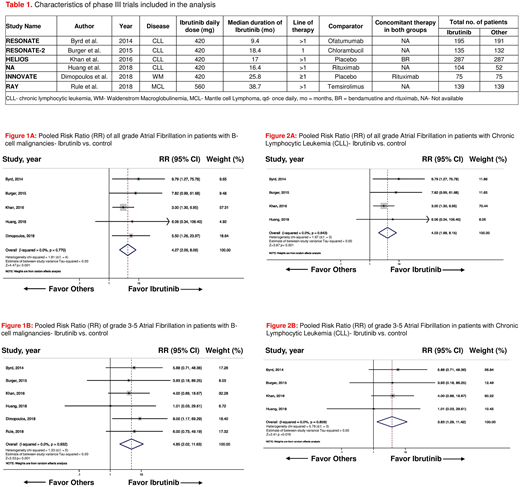
Results: A total of 6 phase III RCTs randomizing 1811 patients (pt; 935 on ibrutinib arms and 876 in the control arms) were included in the final analysis. Four trials were conducted in pts with CLL, one in pts with Waldenstrom macroglobulinemia and the other one in pts with mantle cell lymphoma. Characteristics of these trials are shown in Table 1. In 4 RCTs ibrutinib was compared with an active agent (i.e., ofatumumab, chlorambucil, temsirolimus, and rituximab) and in the other two trials, it was compared with placebo. Ibrutinib was administered as a 1st-line therapy in the RESONATE-2 trial and both as 1st line and in refractory cases in the iNNOVATE trial. The median duration of treatment across studies with ibrutinib was 17.7 months (range 9.4-38.7 months). The pooled risk ratio (RR) for all grade atrial fibrillation was 4.27 [95% confidence interval (CI): 2.26-8.06, p<0.001, I2=0.0%, figure 1A] in patients on ibrutinib across all B-cell malignancies, as compared to patients in the control arms. The overall incidence of high-grade (grade 3-5) AF was also significantly increased in the ibrutinib arms, as compared to control [pooled RR 4.85, 95% CI: 2.02-11.63, p<0.001, I2=0.0%, figure 1B]. In subgroup analysis of trials (n=4) in pts with CLL only, ibrutinib was again associated with a statistically significant increase in risk of all grade and high-grade AF [pooled RR 4.03, 95% CI: 1.99-8.15, p<0.001, I2=0.0%, figure 2A, and pooled RR 3.83, 95% CI: 1.29-11.42, p=0.016, I2=0.0%, figure 2B, respectively]. No publication bias was observed across all the studies included in final analysis.
Conclusion: In agreement with prior reports, this updated meta-analysis confirmed that ibrutinib was associated with a significantly increased risk of all grade AF in pts with B-cell malignancies (RR 4.27). Our analysis shows that the risk of high grade AF (grade3-5) is also significantly increased with ibrutinib (RR 4.85). These pts need to be closely monitored for development of AF to help improve morbidity and mortality.
Short:Takeda Oncology: Consultancy. Maiti:Celgene Corporation: Other: Research funding to the institution.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal